Detection of Catalyst Poisons
Organic molecules, sulphur species and other contaminants in the feed, reactants or intermediate products within chemical processes can affect the activity/efficiency of the costly catalysts even at trace level. Thus there is a strong need to monitor and remove smallest concentrations of them.
A catalyst poison is a substance that reduces the effectiveness of a catalyst in a chemical reaction. Most reactions are irreversible and accumulation of poisonous compounds continuously reduce the facility’s output. The deactivation of heterogeneous catalysts in industrial processes causes a damage of billions of dollars per year due to catalyst replacement and downtimes.
Common compounds, which act as catalyst poisons, are carbon monoxide, halides, cyanides, sulfides, sulfites, phosphites. Likewise, organic molecules such as nitriles, nitro compounds, oximes and nitrogen-containing heterocycles may have a poisonous effect on catalyst materials too. Sulphur species are catalyst poisons especially for processes involving reduced metals when applied as active phase.
A customized version of the GC-IMS made by G.A.S. has proven to be a very useful online monitoring tool to test for a variety of above mentioned species 24/7 on-site at low ppb-level.
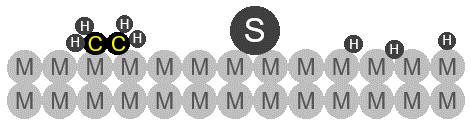
Schematic drawing of sulfur poisoning in ethene (CH4) hydrogenation process. A sulfur atom (S) strongly binds to the catalyst (M) metal surface, reducing the catalytic activity by the following exemplary effects:
-
Sterically blocking adsorption/reaction sites of the catalyst (also inhibiting lateral diffusion)
-
Electronically modification of catalyst surface
Taken from [4]. Copyright 2006, Wiley-Interscience.
[4] Nelson, A.E. (2007), Fundamentals of Industrial Catalytic Processes, 2nd Edition. C. H. Bartholomew and Robert J. Farrauto John Wiley and Sons, Hoboken, NJ, 966 pp., 2006. Can. J. Chem. Eng., 85: 127-128.

 Deutsch
Deutsch English
English Chinese
Chinese